2018 Volume No 36 – pages 44-56
Title: Inhibition of apoptosis exacerbates fatigue-damage tendon injuries in an in vivo rat model |
Authors: R Bell, MA Robles-Harris, M Anderson, D Laudier, MB Schaffler, EL Flatow, N Andarawis-Puri |
Address: Sibley School of Mechanical and Aerospace Engineering, Cornell University, Ithaca, 14850, NY, USA |
E-mail: Na424 at cornell.edu |
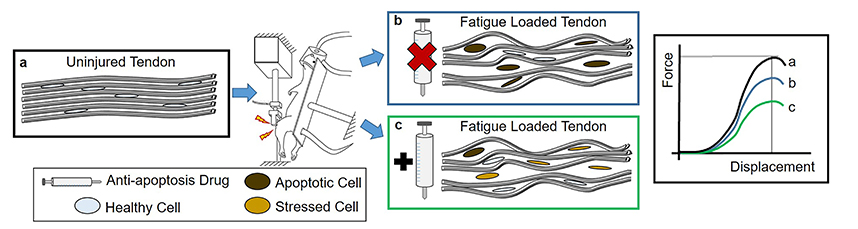
Abstract: Tendinopathy is a common and progressive musculoskeletal disease. Increased apoptosis is an end-stage tendinopathy manifestation, but its contribution to the pathology of the disease is unknown. A previously established in vivo model of fatigue damage accumulation shows that increased apoptosis is correlated with the severity of induced tendon damage, even in early onset of the disease, supporting its implication in the pathogenesis of the disease. Consequently, this study aimed to determine: (1) whether apoptosis could be inhibited after fatigue damage and (2) whether its inhibition could lead to remodeling of the extracellular matrix (ECM) and pericellular matrix (PCM), to ultimately improve the mechanical properties of fatigue-damaged tendons. The working hypothesis was that, despite the low vascular nature of the tendon, apoptosis would be inhibited, prompting increased production of matrix proteins and restoring tendon mechanical properties. Rats received 2 or 5 d of systemic pan-caspase inhibitor (Q-VD-OPh) or dimethyl sulfoxide (DMSO) carrier control injections starting immediately prior to fatigue loading and were sacrificed at days 7 and 14 post-fatigue-loading. Systemic pan-caspase inhibition for 2 d led to a surprising increase in apoptosis, but inhibition for 5 d increased the population of live cells that could repair the fatigue damage. Further analysis of the 5 d group showed that effective inhibition led to an increased population of cells producing ECM and PCM proteins, although typically in conjunction with oxidative stress markers. Ultimately, inhibition of apoptosis led to further deterioration in mechanical properties of fatigue-damaged tendons.
|
Key Words: Cells, tissues, ligament, tendon, extracellular matrix collagens, connective tissues, tendon, cells/tissues-aging/apoptosis, animal models. |
Publication date: July 30th 2018 |
Article download: Pages 44-56 (PDF file) |