2019 Volume No 38 – pages 168-187
Title: Biofabrication of multiscale bone extracellular matrix scaffolds for bone tissue engineering |
Authors: FE Freeman, DC Browe, J Nulty, S Von Euw, WL Grayson, DJ Kelly |
Address: Trinity Centre for Biomedical Engineering, Trinity Biomedical Sciences Institute, Trinity College Dublin, Ireland |
E-mail: KELLYD9 at tcd.ie |
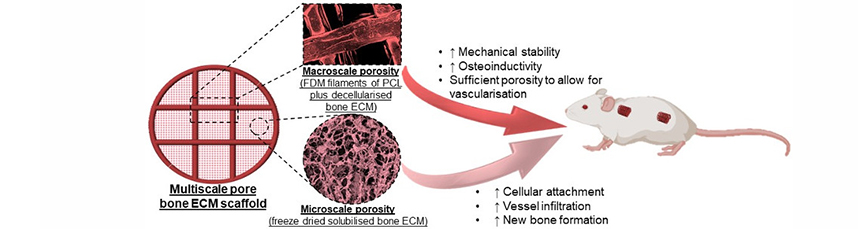
Abstract: Interconnected porosity is critical to the design of regenerative scaffolds, as it permits cell migration, vascularisation and diffusion of nutrients and regulatory molecules inside the scaffold. 3D printing is a promising strategy to achieve this as it allows the control over scaffold pore size, porosity and interconnectivity. Thus, the aim of the present study was to integrate distinct biofabrication strategies to develop a multiscale porous scaffold that was not only mechanically functional at the time of implantation, but also facilitated rapid vascularisation and provided stem cells with appropriate cues to enable their differentiation into osteoblasts. To achieve this, polycaprolactone (PCL) was functionalised with decellularised bone extracellular matrix (ECM), to produce osteoinductive filaments for 3D printing. The addition of bone ECM to the PCL not only increased the mechanical properties of the resulting scaffold, but also increased cellular attachment and enhanced osteogenesis of mesenchymal stem cells (MSCs). In vivo, scaffold pore size determined the level of vascularisation, with a larger filament spacing supporting faster vessel in-growth and more new bone formation. By freeze-drying solubilised bone ECM within these 3D-printed scaffolds, it was possible to introduce a matrix network with microscale porosity that further enhanced cellular attachment in vitro and increased vessel infiltration and overall levels of new bone formation in vivo. To conclude, an “off-the-shelf” multiscale bone-ECM-derived scaffold was developed that was mechanically stable and, once implanted in vivo, will drive vascularisation and, ultimately, lead to bone regeneration. |
Key Words: 3D printing, extracellular matrix, bone, tissue engineering, osteogenesis, mesenchymal stem cells. |
Publication date: October 11th 2019 |
Article download: Pages
168-187 (PDF file) |