Address: MIT, Centre for Biomedical Engineering, 500 Technology Square, Cambridge, MA, 02139, USA.
|
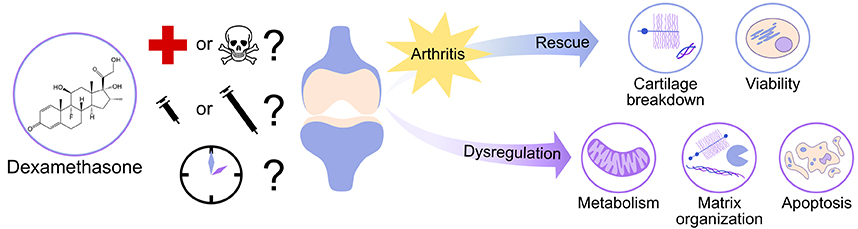
Abstract: While glucocorticoids have been used for over 50 years to treat rheumatoid and osteoarthritis pain, the prescription of glucocorticoids remains controversial because of potentially harmful side effects at the molecular, cellular and tissue levels. One member of the glucocorticoid family, dexamethasone (DEX) has recently been demonstrated to rescue cartilage matrix loss and chondrocyte viability in animal studies and cartilage explant models of tissue injury and post-traumatic osteoarthritis, suggesting the possibility of DEX as a disease-modifying drug if used appropriately. However, the literature on the effects of DEX on cartilage reveals conflicting results on the drug’s safety, depending on the dose and duration of DEX exposure as well as the model system used. Overall, DEX has been shown to protect against arthritis-related changes in cartilage structure and function, including matrix loss, inflammation and cartilage viability. These beneficial effects are not always observed in model systems using initially healthy cartilage or isolated chondrocytes, where many studies have reported significant increases in chondrocyte apoptosis. It is crucially important to understand under what conditions DEX may be beneficial or harmful to cartilage and other joint tissues and to determine potential for safe use of this glucocorticoid in the clinic as a disease-modifying drug.
|
Key Words: Cartilage-general, cells/tissues-injury, cells/tissues-cartilage, ECM-general, ECM-proteoglycans, cells/tissues-proliferation, cells/tissues chondrocytes.
|

