2020 Volume No 39 – pages 211-226
Title: Crosslinker concentration controls TGFβ-3 release and annulus fibrosus cell apoptosis in genipin-crosslinked fibrin hydrogels |
Authors: CJ Panebianco, TJ DiStefano, B Mui, WW Hom, JC Iatridis |
Address: Leni and Peter W. May Department of Orthopaedics, Icahn School of Medicine at Mount Sinai, New York, NY, USA |
E-mail: james.iatridis at mssm.edu |
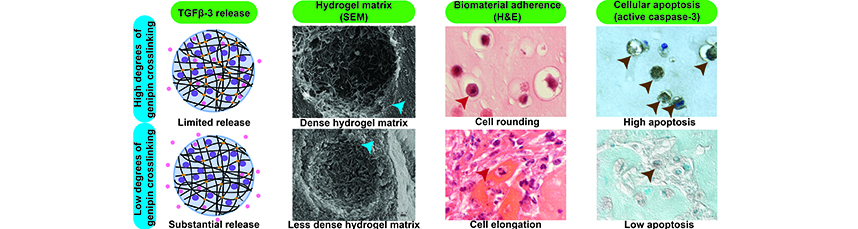
Abstract: Back pain is a leading cause of global disability associated with intervertebral disc (IVD) pathologies. Discectomy alleviates disabling pain caused by IVD herniation without repairing annulus fibrosus (AF) defects, which can cause accelerated degeneration and recurrent pain. Biological therapies show promise for IVD repair but developing high-modulus biomaterials capable of providing biomechanical stabilisation and delivering biologics remains an unmet challenge. The present study identified critical factors and developed an optimal formulation to enhance the delivery of AF cells and transforming growth beta-3 (TGFβ-3) in genipin-crosslinked fibrin (FibGen) hydrogels. Part 1 showed that AF cells encapsulated in TGFβ-3-supplemented high-modulus FibGen synthesised little extracellular matrix (ECM) but could release TGFβ-3 at physiologically relevant levels. Part 2 showed that AF cells underwent apoptosis when encapsulated in FibGen, even after reducing fibrin concentration from 70 to 5 mg/mL. Mechanistic experiments, modifying genipin concentration and integrin binding site presence demonstrated that genipin crosslinking caused AF cell apoptosis by inhibiting cell-biomaterial binding. Adding integrin binding sites with fibronectin partially rescued apoptosis, indicating genipin also caused acute cytotoxicity. Part 3 showed that FibGen formulations with 1 mg/mL genipin had enhanced ECM synthesis when supplemented with fibronectin and TGFβ-3. In conclusion, FibGen could be used for delivering biologically active compounds and AF cells, provided that formulations supplied additional sites for cell-biomaterial binding and genipin concentrations were low. Results also highlighted a need for developing strategies that protect cells against acute crosslinker cytotoxicity to overcome challenges of engineering high-modulus cell carriers for musculoskeletal tissues that experience high mechanical demands. |
Key Words: Intervertebral disc, annulus fibrosus repair, injectable biomaterials, hydrogel, cell delivery, growth factor delivery, fibrin, genipin crosslinking, apoptosis, musculoskeletal tissue engineering. |
Publication date: May 12th 2020 |
Article download: Pages 211-226 (PDF file) |