2021 Volume No 41 – pages 153-169
Title: Priming as a strategy to overcome detrimental pH effects on cells for intervertebral disc regeneration |
Authors: J Gansau, CT Buckley |
Address: Trinity Centre for Biomedical Engineering, Trinity Biomedical Sciences Institute, Trinity College Dublin, Dublin, Ireland |
E-mail: conor.buckley at tcd.ie |
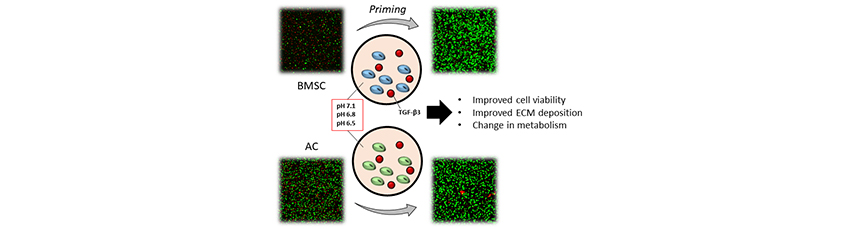
Abstract: Disc disease is characterised by degeneration of the nucleus pulposus (NP), the central gelatinous tissue of the intervertebral disc (IVD). As degeneration progresses, the microenvironment of the IVD becomes more hostile (i.e. decrease in oxygen, glucose and pH), providing a significant challenge for regeneration using cell-based therapies. Tissue engineering strategies such as priming cells or micro tissues with growth factors prior to implantation may overcome some of these issues by providing a pre-formed protective niche composed of extracellular matrix. The present study investigated the effect of priming on bone-marrow-derived stem cells (BMSCs) and articular chondrocytes (ACs) using transforming growth factor β3 (TGF-β3), cultured at different pH levels (pH 7.1, 6.8 and 6.5) representative of the in vivo disc microenvironment. Low pH was found to have a detrimental effect on both cell viability and matrix accumulation, which could be mitigated by priming cells using TGF-β3. Investigating the activation of the transmembrane acid-sensing ion channels (ASIC-1 and -3) showed an increased expression of ASIC-1 in BMSCs and ASIC-3 in ACs at lower pH levels post-priming. Metabolic activity in terms of lactic acid production was also found to be affected significantly by priming, whereas oxygen and glucose consumptions did not change considerably. Overall, the study demonstrated that cells could be equipped to sustain the harsh environment of the IVD and promote accumulation of NP-like matrix through priming. Such an approach may open new avenues to engineer tissues capable of sustaining challenging microenvironments such as those found in the IVD. |
Key Words: Acid-sensing ion channel, articular chondrocytes, bone-marrow-derived stem cells, hydrogel, metabolism, nucleus pulposus, pre-culture, transforming growth factor β3. |
Publication date: February 10th 2021 |
Article download: Pages
153-169 (PDF file) |