2021 Volume No 41 – pages 216-232
Title: Pulsed electromagnetic fields synergize with graphene to enhance dental pulp stem cell-derived neurogenesis by selectively targeting TRPC1 channels |
Authors: TT Madanagopal, YK Tai, SH Lim, CHH Fong, T Cao, V Rosa, A Franco-Obregón |
Address: Department of Surgery, Yong Loo Lin School of
Medicine, National University of Singapore, NUHS Tower Block, Level 8, IE Kent Ridge Road, Singapore,
119228 Singapore |
E-mail: suraf at nus.edu.sg |
Abstract: Conventional root canal treatment replaces the infected pulp with defined materials. Alternative cell-based
tissue engineering strategies aim to regenerate a fully functional pulp within the root canal. Despite recent
advances in this area, however, the regeneration of an innervated pulp remains a major challenge in the
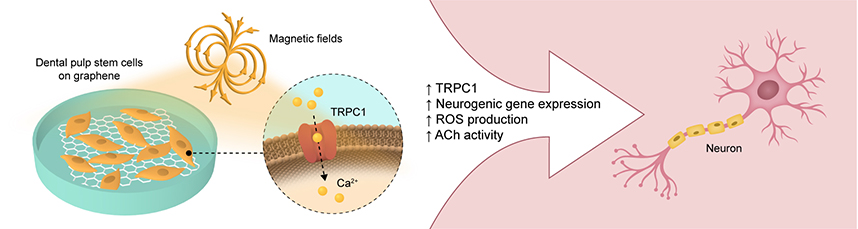
field. Both graphene (2DG) and pulsed electromagnetic fields (PEMFs) independently have been shown
to promote diverse cellular developmental programs. The present study showed that 2DG promoted the
neurogenic induction of human dental pulp stem cells (hDPSCs) by upregulating and accelerating the
expression of mature neuronal markers. Notably, 2DG induced the highest expression of transient receptor
potential canonical cation channel type 1 (TRPC1) during early neurogenesis. As brief PEMF exposure
promotes in vitro differentiation by activating a TRPC1-mitochondrial axis, an opportunity to combine
2DG with developmentally targeted PEMF exposure for synergistic effects was realizable. Neurogenic gene
expression, neurotransmitter release, and reactive oxygen species (ROS) production were greatly enhanced
by a brief (10 min) and low amplitude (2 mT) PEMF exposure timed to coincide with the highest TRPC1
expression from hDPSCs on 2DG. In contrast, hDPSCs on glass were less responsive to PEMF exposure. The
capacity of PEMFs to promote neurogenesis was precluded by the administration of penicillin/streptomycin,
mirroring previous studies demonstrating that aminoglycoside antibiotics block TRPC1-mediated calcium
entry and verifying the contribution of TRPC1 in this form of magnetoreception. Hence, graphene created
a more conducive environment for subsequent PEMF-stimulated neurogenic induction of hDPSCs through
their mutual capacity to activate TRPC1with subsequent ROS production. |
Key Words: Pulsed electromagnetic fields, mitohormesis, tissue engineering, nanomaterial, pulp regeneration.
|
Publication date: March 1st 2021 |
Article download: Pages
216-232 (PDF file) |