2022 Volume No 43 – pages 112-129
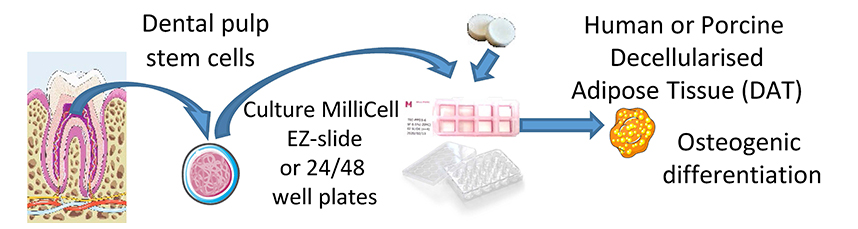
Title: Osteogenic differentiation of human dental pulp stem cells in decellularised adipose tissue solid foams |
Authors: J Luzuriaga, P García-Gallastegui, N García-Urkia, JR Pineda, I Irastorza, F-J Fernandez-San-Argimiro, N Briz, B Olalde, F Unda, I Madarieta, G Ibarretxe |
Address: Department of Cell Biology and Histology, Faculty of Medicine and Nursing, University of the Basque Country, UPV/EHU, Leioa, 48940, Bizkaia, Spain |
E-mail: gaskon.ibarretxe at ehu.eus |
Abstract: 3D cell culture systems based on biological scaffold materials obtainable from both animal and human tissues constitute very interesting tools for cell therapy and personalised medicine applications. The white adipose tissue (AT) extracellular matrix (ECM) is a very promising biomaterial for tissue engineering due to its easy accessibility, malleability and proven biological activity. In the present study, human dental pulp stem cells (hDPSCs) were combined in vitro with ECM scaffolds from porcine and human decellularised adipose tissues (pDAT, hDAT) processed as 3D solid foams, to investigate their effects on the osteogenic differentiation capacity and bone matrix production of hDPSCs, compared to single-protein-based 3D solid foams of collagen type I and conventional 2D tissue-culture-treated polystyrene plates. pDAT solid foams supported the osteogenic differentiation of hDPSCs to similar levels to collagen type I, as assessed by alkaline phosphatase and alizarin red stainings, reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR) and osteocalcin/bone gamma-carboxyglutamate protein (BGLAP) immunostaining. Interestingly, hDAT solid foams showed a markedly lower capacity to sustain hDPSC osteogenic differentiation and matrix calcification and a higher capacity to support adipogenesis, as assessed by RT-qPCR and oil red O staining. White ATs from both human and porcine origins are relatively abundant and available sources of raw material to obtain high quality ECM-derived biomedical products. These biomaterials could have promising applications in tissue engineering and personalised clinical therapy for the healing and regeneration of lesions involving not only a loss of calcified bone but also its associated soft non-calcified tissues. |
Keywords: Dental pulp stem cells, adipose tissue, bone tissue, extracellular matrix, decellularisation, solid foam, osteogenic differentiation, mineralisation. |
Publication date: March 21st 2022 |
Article download: Pages
112-129 (PDF file) |