2024 Volume No 47 – pages 219-237
Title: Emerging role of hypertrophic chondrocytes in tissue regeneration and fracture healing: a narrative review |
Authors: DY Zhu, G Li, HY Fang, YS Gao |
Address: Division of Hip Surgery, Department of Orthopedic Surgery, Shanghai Sixth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, 200233 Shanghai, China |
E-mail: gaoyoushui at sjtu.edu.cn |
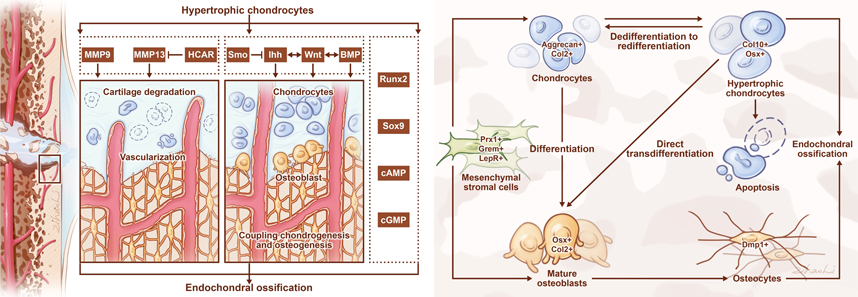
Abstract: Fracture healing is a complex event that involves the coordination of various different processes, including intramembranous and endochondral bone formation. When facing fracture nonunion or delayed union, few organizational engineering structures can achieve the desired results. The main reason for this is that they cannot recapitulate the cellular morphology, biology, and mechanical functions of natural tissues. Ten years ago, the term Development Engineering was coined to refer to the use of developmental processes as a blueprint for designing and developing engineered live implants. Different sources of cells have been used as seed cells in developmental engineering. Among them, hypertrophic chondrocytes have attracted worldwide attention. Hypertrophic chondrocytes are the terminal state of growth plate chondrocytes, leading to degenerative maturation. Hypertrophic chondrocytes mediate crosstalk by regulating cell-matrix degradation, vascularization, osteoclast recruitment, and osteoblast differentiation. Furthermore, hypertrophic chondrocytes can transdifferentiate into osteoprogenitors and mature osteoblasts, and directly promote woven bone formation. In summary, elucidating the role of hypertrophic chondrocytes will contribute to understand of the physiological mechanism of fracture healing, research and development of novel therapeutic modes of developmental engineering, and further promotion of fracture healing. |
Keywords: Hypertrophic chondrocytes, fracture healing, tissue regeneration, endochondral bone formation, developmental engineering, transdifferentiation. |
Publication date: June 14th 2024 |
Copyright policy: © 2024 The Author(s). Published by Forum Multimedia Publishing, LLC. This article is distributed in accordance with Creative Commons Attribution Licence (http://creativecommons.org/licenses/by/4.0/). |
Article download: Pages 219-237 (PDF file) |