2024 Volume No 48 – pages 137-150
Title: Mechanical load rescues injury-induced skeletal muscle fibrosis through macrophage polarization |
Authors: HW Liu, SG Yuan, K Zheng, GF Liu, Y Xie, XL Huang, BF Ye, L Yin, YK Li, JH Li |
Address: Clinical Research Center, Department of Orthopaedic, Panzhihua Central Hospital, 617000 Panzhihua, Sichuan, China; School of Traditional Chinese Medicine, Southern Medical University, 510000 Guangzhou, Guangdong, China; Department of Traditional Chinese Orthopedics and Traumatology, Center for Orthopedic Surgery, The Third Affiliated Hospital, Southern Medical University, 510000 Guangzhou, Guangdong, China |
E-mail: yinli2913 at 163.com; ortho at smu.edu.cn; lijunhua929 at smu.edu.cn |
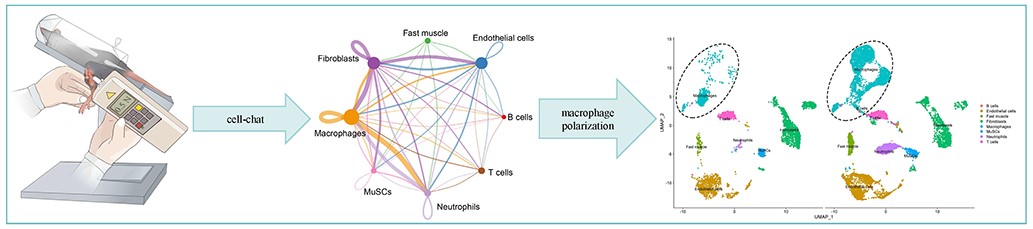
Abstract: Background: Current therapeutic approaches for skeletal muscle fibrosis are insufficient. We hypothesize that mechanical load (ML) could mitigate muscle fibrosis by suppressing inflammation. Methods: We utilized ML to treat cardiotoxin (CTX) injury-induced skeletal muscle fibrosis in C57BL6/J mice. We analyzed the underlying mechanism by integrating single-cell RNA sequencing (scRNA-seq) with molecular techniques. Hematoxylin and eosin staining and Masson’s trichrome staining were used to assess skeletal muscle fibrosis. An enzyme-linked immunosorbent assay was used to detect inflammatory cytokine levels in serum. scRNA-seq, immunofluorescence, and Western blot were performed to determine cellular and molecular outcome changes. Results: After seven days of ML intervention, compared with CTX injury-induced fibrosis mice, ML significantly reduced pro-inflammatory cytokines interleukin (IL)-1β and IL-6 expression and increased anti-inflammatory cytokines transform growth factor (TGF)-β and IL-10 expression levels (all p < 0.05). Meanwhile, ML inhibited the expression of the fibrosis signaling pathway TGF-β1/Smad3 and decreased hyperplasia of fibrosis tissue in skeletal muscle (all p < 0.05). Next, scRNA-seq detected that M1 macrophage numbers, activity, and communication capacity are maximum increased in fibrotic skeletal muscle among B cells, endothelial cells, fast muscle cells, fibroblasts, skeletal muscle satellite cells, neutrophils, and T cells. However, ML significantly suppresses the expression of CD68+ M1 macrophage and p65 in the nuclear factor kappa-B (NF-κB) pathway and promotes the CD206+ M2 macrophage and peroxisome proliferator-activated receptor (PPAR)-γ expression levels in fibrotic skeletal muscle (all p < 0.05). Conclusion: ML can attenuate fibrosis in the injured skeletal muscle of mice by prompt polarization of M1 macrophages into M2 macrophages. |
Keywords: Mechanical load, skeletal muscle fibrosis, macrophage, polarization, inflammation. |
Publication date: 22nd November 2024 |
Copyright policy: © 2024 The Author(s). Published by Forum Multimedia Publishing, LLC. This article is distributed in accordance with Creative Commons Attribution Licence (http://creativecommons.org/licenses/by/4.0/). |
Article download: Pages 137-150 (PDF file) |