2025 Volume No 49 – pages 55-70
Title: Melatonin promotes tendon-derived stem cells differentiation and inhibits oxidative stress in trauma-induced heterotopic ossification |
Authors: J Liu, WS Zhang, QH Chen, MY He, YY Xian, SY Le, YT Jiang, J Zhang, S Chen, L Wang |
Address: Department of Orthopedics, The Third Affiliated Hospital, Southern Medical University, 510630 Guangzhou, Guangdong, China; Department of pediatric orthopedics, Shantou University Guangzhou Huaxin Orthopedic Hospital, 510000 Guangzhou, Guangdong, China |
E-mail: chenshu1228 at 163.com; liang091 at aliyun.com |
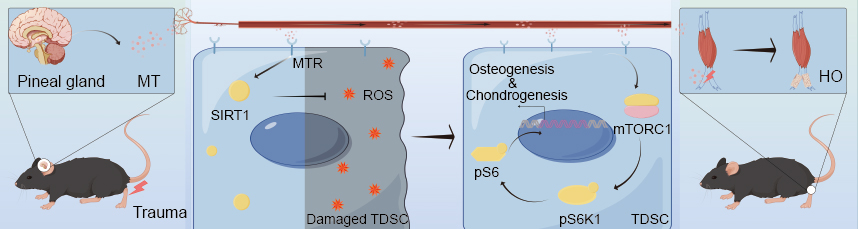
Abstract: Background: Heterotopic ossification (HO) is a common complication of muscle and tendon injury, but its underlying mechanism is currently unclear. Melatonin (MT), a hormone mainly secreted by the pineal gland, plays a key role in the pathogenesis of skeletal diseases. This study aimed to investigate the effects and potential mechanisms of melatonin on the differentiation of tendon-derived stem cells (TDSC) and development of tendon HO. Methods: We employed in vivo and in vitro assays to assess the effects of melatonin and its receptors on TDSC differentiation, specifically focusing on chondrogenic and osteogenic pathways involved in ectopic ossification. Additionally, we investigated the activation of the mTOR complex 1 (mTORC1) pathway by melatonin and its impact on chondrogenesis and osteogenesis in TDSC. The influence of antioxidant enzyme expression and reactive oxygen species (ROS) modulation was analyzed, with a particular focus on Sirtuin 1 (SIRT1) activation, as a mechanism to mediate antioxidative effects and regulate osteogenic differentiation. Results: Our findings demonstrate that melatonin and its receptors significantly contribute to chondrogenesis and osteogenesis during ectopic ossification. Melatonin was observed to activate the mTORC1 pathway, which promoted chondrogenic and osteogenic differentiation in TDSC, accelerating the progression of HO. Inhibiting the mTORC1 pathway reduced melatonin-induced HO in a mouse model, indicating the pathway’s essential role in this process. Furthermore, melatonin enhanced the expression of antioxidant enzymes, thereby reducing ROS levels and enhancing TDSC osteogenic differentiation potential through SIRT1 activation. Suppression of SIRT1 activation in vivo mitigated HO progression, highlighting its role in oxidative stress regulation and osteogenic differentiation. Conclusions: These findings suggest that melatonin accelerates HO by activating the mTORC1 pathway to promote chondrogenic and osteogenic differentiation of TDSC. Additionally, melatonin’s antioxidative effect, mediated through SIRT1 activation, preserves TDSC osteogenic potential in the early stages of injury. This study identifies mTORC1 and SIRT1 as potential therapeutic targets for the prevention and management of HO, offering new insights into the molecular mechanisms of HO development and treatment strategies. |
Keywords: Melatonin, mTORC1 pathway, oxidative stress, SIRT1. |
Publication date: 20th February 2025 |
Copyright policy: © 2025 The Author(s). Published by Forum Multimedia Publishing, LLC. This article is distributed in accordance with Creative Commons Attribution Licence (http://creativecommons.org/licenses/by/4.0/). |
Article download: Pages 55-70 (PDF file) |