2025 Volume No 50 – pages 68-83
Title: A preclinical assessment of rapidly isolated chondro-progenitor cells from the infrapatellar fat pad for single-stage articular cartilage regeneration |
Authors: DC Browe, OR Mahon, PJ Díaz-Payno, R Burdis, FE Freeman, JM Nulty, P Pitacco, G Gonnella, A Dunne, CJ Moran, PAJ Brama, CT Buckley, DJ Kelly |
Address: Trinity Centre for Biomedical Engineering, Trinity Biomedical Sciences Institute, Trinity College Dublin, D02 R590 Dublin, Ireland |
E-mail: kellyd9 at tcd.ie |
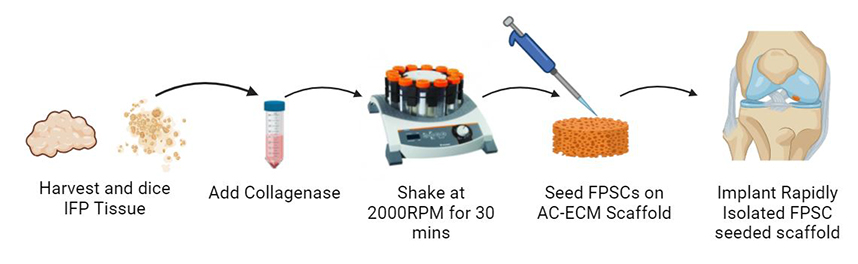
Abstract: Background: Clinically approved cell-based cartilage repair products are associated with multiple surgeries and high cost. There is therefore an unmet clinical need for a cell-based approach that is efficacious and cost effective that can be performed intraoperatively as a single-stage procedure. Methods: Here, we developed a novel methodology to rapidly isolate chondro-progenitor cells from human infrapatellar fat pad tissue that reduced the isolation time from over three hours to under one hour while still being able to yield clinically relevant cell numbers. Cell yields and biochemical characteristics were compared with conventionally isolated control cells. In vitro assays evaluated cartilage-specific matrix deposition across multiple human donors. Constructs combining rapidly isolated cells and articular cartilage extracellular matrix-derived scaffolds were implanted into caprine cartilage defects and analyzed after six months in vivo. Results: These rapidly isolated cells contained a larger fraction of colony-forming cells than conventionally isolated control cells, and an analysis of surface marker expression revealed a higher percentage of CD44+ (a putative progenitor cell marker) cells in this group. Furthermore, these rapidly isolated cells supported higher levels of cartilage-specific matrix deposition in vitro for multiple human donors. We then seeded such rapidly isolated cells onto cartilage extracellular matrix (ECM) derived scaffolds and immediately (i.e., no in vitro pre-culture) implanted these constructs into caprine cartilage defects. After 6 months in vivo, treatment with this cell and scaffold combination typically generated a repair tissue that was rich in glycosaminoglycans and type II collagen, with biomimetic collagen fiber alignment and lubricin expression in the superficial zone, which was generally not observed in defects treated with microfracture. However, in this model the addition of the rapidly isolated cells did not result in any significant improvement in repair metrics compared to treatment with the extracellular matrix scaffold alone. Conclusions: While this rapidly isolated cell population processes a strong chondrogenic potential in vitro, further work is required to identify clinical scenarios where it will provide clear therapeutic benefits. |
Keywords: Cartilage repair, articular cartilage, stem cell therapy, tissue engineering, regenerative medicine, biomaterials. |
Publication date: 24th April 2025 |
Copyright policy: © 2025 The Author(s). Published by Forum Multimedia Publishing, LLC. This article is distributed in accordance with Creative Commons Attribution Licence (http://creativecommons.org/licenses/by/4.0/). |
Article download: Pages 68-83 (PDF file) |