2025 Volume No 50 – pages 87-108
Title: Lung cancer-associated mesenchymal stem cells mediate chemoresistance and malignant progression of lung cancer through activating HIF-1α |
Authors: LB Pang, J Li, ZW Zhang, CQ Zhang |
Address: Cheeloo College of Medicine, Shandong University, 250013 Jinan, Shandong, China; Department of Pulmonary and Critical Care Medicine, Shandong Provincial ENT Hospital, 250000 Jinan, Shandong, China; Department of Radiology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, 250021 Jinan, Shandong, China |
E-mail: freezcq66 at 163.com; sduzzw at 163.com |
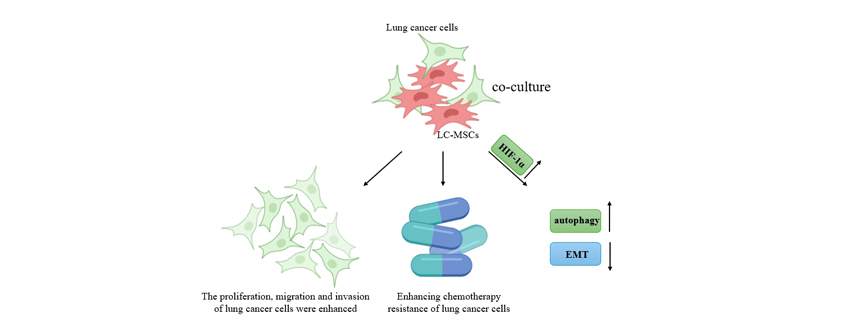
Abstract: Background: The high mortality of lung cancer is mainly attributed to not only late detection and diagnosis but also strong invasion and the potential development of chemoresistance. This study investigated the effect and underlying mechanisms of mesenchymal stem cells (MSCs) isolated from lung cancer tissues (LC-MSCs) on lung cancer progression and chemoresistance. Methods: LC-MSCs were isolated using tissue culture method. They were characterized and verified through microscopic observation via flow cytometry (for determining MSC expression markers) and testing their ability of differentiation into adipose cells, osteocytes, or chondrocytes. Then, lung cancer A549 and H1299 cell lines cultured alone or co-cultured with LC-MSCs were used for exploring how LC-MSCs affect the progression of lung cancer cells. Temozolomide (TMZ) was administrated on differently cultured cells to determine how LC-MSCs affect the chemoresistance of lung cancer cells. Cell proliferation was evaluated by cell counting kit-8 assay, and cell cycle distribution was assessed by flow cytometry. Cell invasion, migration, and apoptosis were determined by Transwell assay, wound healing assay, and flow cytometry, respectively. Reverse transcription quantitative polymerase chain reaction, Western blot, and immunofluorescence were used to verify the upregulation of hypoxia-inducible factor-1α (HIF-1α) by LC-MSCs, and HIF-1α expression was mediated by transfection to investigate the effect of HIF-1α on lung cancer cell progression and chemoresistance. The effects of LC-MSCs and HIF-1α on autophagy-related LC3-II/I ratio, Beclin-1, p62, and epithelial-mesenchymal transition (EMT)-related N-cadherin and E-cadherin were measured. Nude BALB/c mice were subcutaneously injected with lung cancer cells or LC-MSCs co-cultured with lung cancer cells. They were also administrated with or without TMZ. The tumor volume, tumor weight, and levels of HIF-1α, LC3-II/I ratio, Beclin-1, p62, E-cadherin and N-cadherin, proliferation (Ki-67 immunohistochemistry), and apoptosis (terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay) of the formed lung cancer tumor tissues were measured. Results: Under or under no TMZ administration, LC-MSCs promoted cell proliferation, migration, and invasion and inhibited cell apoptosis (p < 0.01). When co-cultured with LC-MSCs, HIF-1α was more expressed in cells (p < 0.01). Under or under no TMZ administration, HIF-1α increased proliferation, migration, invasion, autophagy, and EMT and decreased the apoptosis of cells co-cultured with LC-MSCs (p < 0.01). In mice treated with TMZ, LC-MSCs and HIF-1α promoted tumor growth in terms of volume and weight, proliferation, autophagy, and EMT in tumor cells and inhibited cell apoptosis (p < 0.01). Conclusions: LC-MSCs promote the malignant progression of lung cancer and enhance cancer cell resistance to chemotherapy through upregulating HIF-1α expression, thus providing new targets of LC-MSCs and HIF-1α for lung cancer treatment. |
Keywords: Mesenchymal stem cells, lung cancer, HIF-1α, chemoresistance, temozolomide. |
Publication date: 24th April 2025 |
Copyright policy: © 2025 The Author(s). Published by Forum Multimedia Publishing, LLC. This article is distributed in accordance with Creative Commons Attribution Licence (http://creativecommons.org/licenses/by/4.0/). |
Article download: Pages 87-108 (PDF file) |